The Mechanism of Denaturation and the Unfolded State of the α-Helical Membrane-Associated Protein Mistic
21-Mar-2013
J. Am. Chem. Soc.,, 2013, DOI: 10.1021/ja408644f, 135 (50), pp 18884–18891 published on 21.03.2013
J. Am. Chem. Soc., online article
J. Am. Chem. Soc., online article
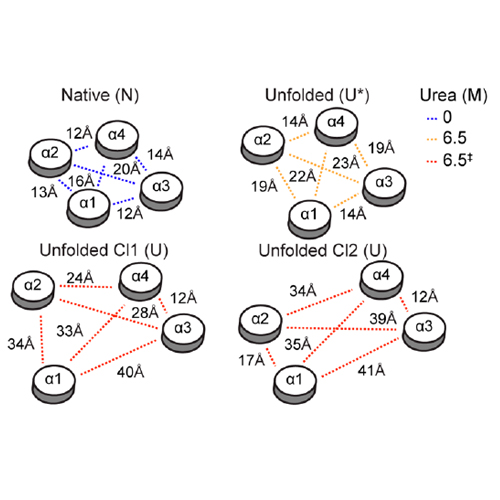
In vitro protein-folding studies using chemical denaturants such as urea are indispensible in elucidating the forces and mechanisms determining the stability, structure, and dynamics of water-soluble proteins. By contrast, α-helical membrane-associated proteins largely evade such approaches because they are resilient to extensive unfolding. We have used optical and NMR spectroscopy to provide an atomistic-level dissection of the effects of urea on the structure and dynamics of the α-helical membrane-associated protein Mistic as well as its interactions with detergent and solvent molecules. In the presence of the zwitterionic detergent lauryl dimethylamine oxide, increasing concentrations of urea result in a complex sequence of conformational changes that go beyond simple two-state unfolding. Exploiting this finding, we report the first high-resolution structural models of the urea denaturation process of an α- helical membrane-associated protein and its completely unfolded state, which contains almost no regular secondary structure but nevertheless retains a topology close to that of the folded state.