Structural and Mechanistic Implications of Metal-Binding in the Small Heat-Shock Protein αB-Crystallin
06-Jan-2012
Journal of Biological Chemistry, 2012, DOI 10.1074/jbc.M111.309047, VOL. 287, NO. 2, pp. 1128–1138, published on 06.01.2012
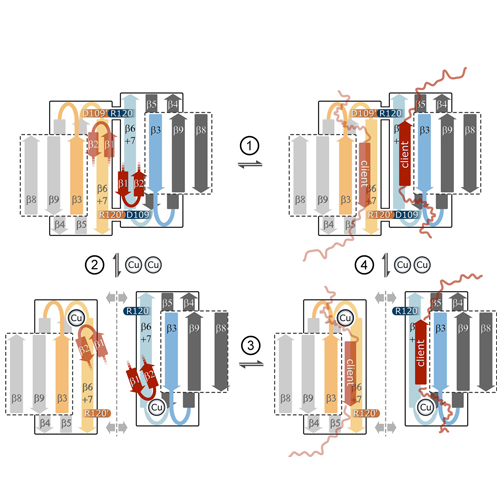
The human small heat-shock protein alphaB-crystallin (alphaB) rescues misfolded proteins from irreversible aggregation during cellular stress. Binding of Cu(II) was shown to modulate the oligomeric architecture and the chaperone activity of alphaB. However, the mechanistic basis of this stimulation is so far not understood. We provide here first structural insights into this Cu(II)-mediated modulation of chaperone function using NMR spectroscopy and other biophysical approaches. We show that the alpha-crystallin domain is the elementary Cu(II)-binding unit specifically coordinating one Cu(II) ion with picomolar binding affinity. Putative Cu(II) ligands are His83, His104, His111, and Asp109 at the dimer interface. These loop residues are conserved among different metazoans, but also for human alphaA-crystallin, HSP20, and HSP27. The involvement of Asp109 has direct implications for dimer stability, because this residue forms a salt bridge with the disease-related Arg120 of the neighboring monomer. Furthermore, we observe structural reorganization of strands beta2-beta3 triggered by Cu(II) binding. This N-terminal region is known to mediate both the intermolecular arrangement in_B oligomers and the binding of client proteins. In the presence of Cu(II), the size and the heterogeneity of alphaB multimers are increased. Atthesametime, Cu(II) increases the chaperone activity of alphaB toward the lens-specific protein betaL-crystallin. We therefore suggest that Cu(II) binding unblocks potential client binding sites and alters quaternary dynamics of both the dimeric building block as well as the higher order assemblies of alphaB.