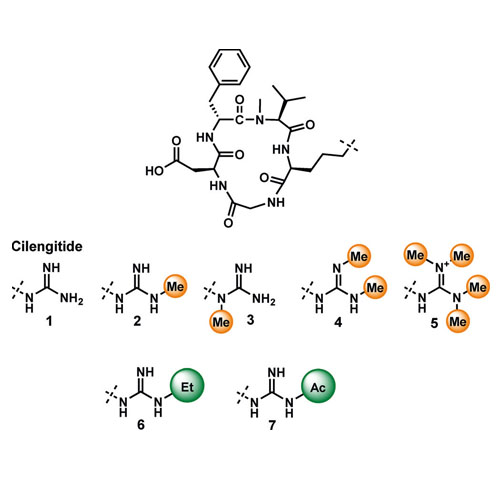
Small Cause, Great Impact: Modification of the Guanidine Group in the RGD Motif Controls Integrin Subtype Selectivity
09-Dec-2015
Angew. Chem. Int. Ed., Volume 55, Issue 4, Pages 1540–1543, DOI: 10.1002/anie.201508713
Angew. Chem. Int. Ed., online article
Due to its unique role as a hydrogen-bond donor and its positive charge, the guanidine group is an important pharmacophoric group and often used in synthetic ligands. The chemical modification of the guanidine group is often considered to destroy its function. Herein, we show that the N-methylation, N-alkylation, or N-acylation of the guanidine group can be used to modify the receptor subtype specificity of the integrin ligand cilengitide. Using the αvβ6/α5β1-biselective ligand c(isoDGRkphg) and the αvβ6-specific ligand c(FRGDLAFp(NMe)K(Ac) as examples, we show that the binding affinities of the ligands can be fine-tuned by this method to enhance the selectivity for αvβ6. Furthermore, we describe a new strategy for the functionalization of integrin ligands. By introducing longer N-alkylguanidine and N-acylguanidine groups, we are able to simultaneously identify a hitherto unknown anchoring point and enhance the subtype selectivity of the ligand.