Seeing Cisplatin - Discerning how an anticancer drug meddles with replication, transcription
12-Nov-2007
Chemical & Engineering News, 2007, Vol. 85, Nr. 46, 13 published on 12.11.2007
Chemical & Engineering News, online article
Chemical & Engineering News, online article
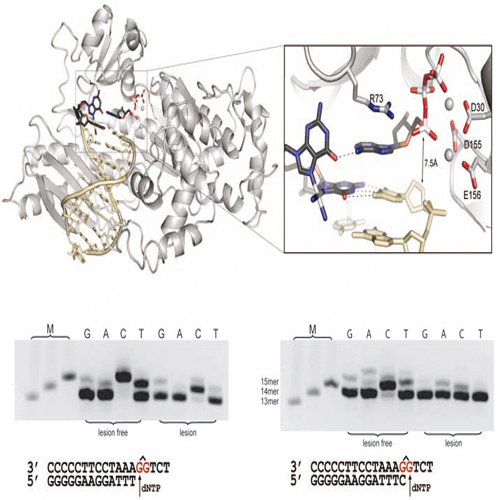
CISPLATIN HAS BEEN USED to treat cancer for decades. But the drug's detailed molecular interactions with the enzymes it aims to interrupts have remained obscure. In two separate papers, researchers are now reporting the first crystal structures of cisplatin complexed with two key enzymes. "For the first time, we are getting deep insight on what goes on at the atomic level with cisplatin and cellular machinery," says Thomas Carell, a protein chemist at the University of Munich. Cisplatin creates bulky platinum adducts with pairs of guanidine bases that are side-by-side or one base apart on genomic DNA. The extra bulk blocks most DNA polymerases that slide along DNA when they copy the genetic code during cell division. The adducts also obstruct RNA polymerases, which transcribe DNA to make proteins. But no structures of cisplatin in complex with either DNA or RNA polymerases have come to light until now. In one report, Carell and Karl-Peter Hopfner announce the structure of a renegade DNA polymerase called Pol η. Normal DNA polymerases get stalled by cisplatin because their grip on DNA is so tight that there's no room for adduct riffraff. But Pol η manages to replicate over unwelcome DNA adducts. Original Science article, Science 2007, 318, 967 See also under www.cipsm.de