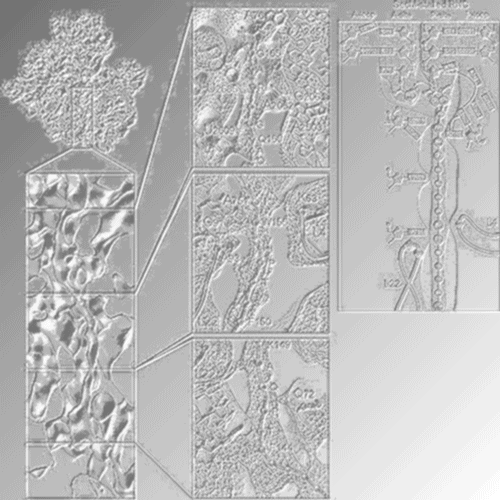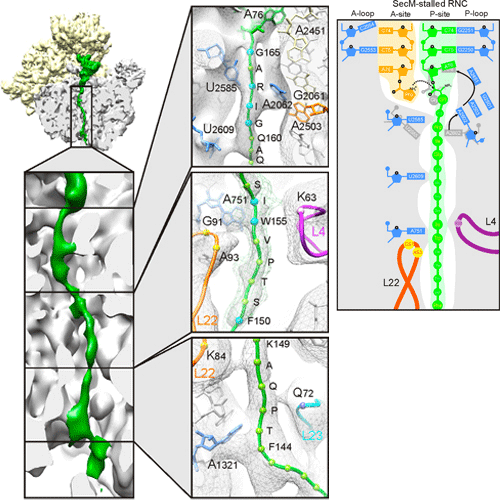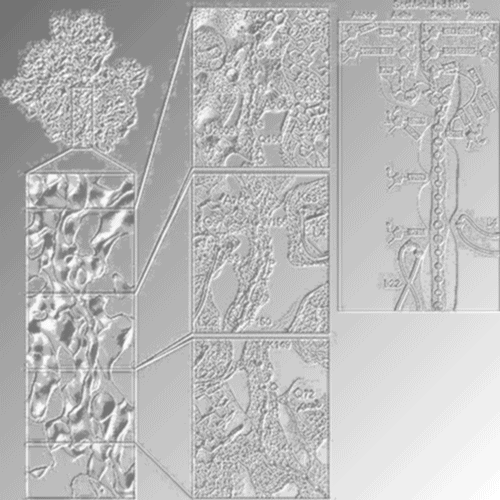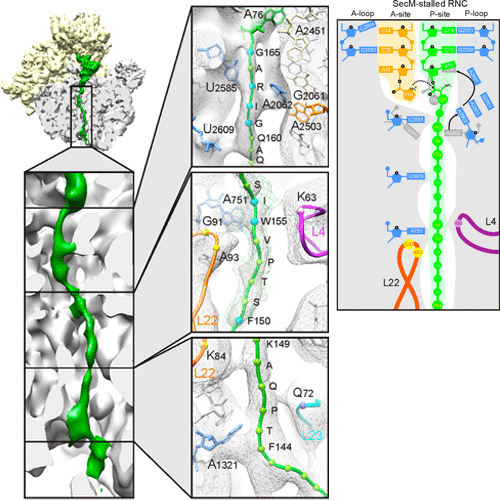
SecM-Stalled Ribosomes Adopt an Altered Geometry at the Peptidyl Transferase Center
18-Jan-2011
PLOS Biology, 2011, doi:10.1371/journal.pbio.1000581, 10 published on 18.01.2011
PLOS Biology, online article
PLOS Biology, online article
As nascent polypeptide chains are synthesized, they pass through a tunnel in the large ribosomal subunit. Interaction between specific nascent chains and the ribosomal tunnel is used to induce translational stalling for the regulation of gene expression. One well-characterized example is the Escherichia coli SecM (secretion monitor) gene product, which induces stalling to up-regulate translation initiation of the downstream secA gene, which is needed for protein export. Although many of the key components of SecM and the ribosomal tunnel have been identified, understanding of the mechanism by which the peptidyl transferase center of the ribosome is inactivated has been lacking. Here we present a cryo-electron microscopy reconstruction of a SecM-stalled ribosome nascent chain complex at 5.6 A°. While no cascade of rRNA conformational changes is evident, this structure reveals the direct interaction between critical residues of SecM and the ribosomal tunnel. Moreover, a shift in the position of the tRNA–nascent peptide linkage of the SecM-tRNA provides a rationale for peptidyl transferase center silencing, conditional on the simultaneous presence of a Pro-tRNAPro in the ribosomal A-site. These results suggest a distinct allosteric mechanism of regulating translational elongation by the SecM stalling peptide.