Rational Design of Highly Active and Selective Ligands for the alpha-5-beta-1 Integrin Receptor
09-Jun-2008
ChemBioChem, 2008, 9, 1397-1407 published on 09.06.2008
ChemBioChem; online article
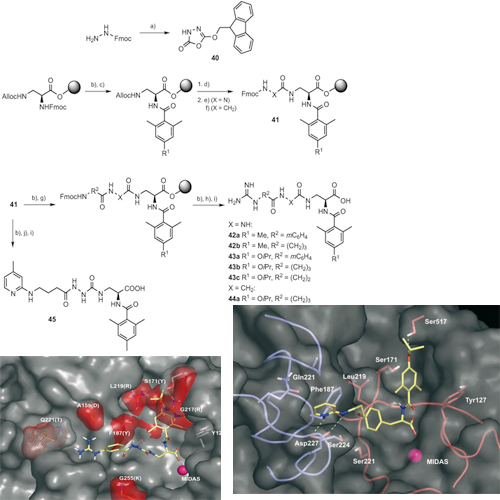
The inhibition of integrin function is a major challenge in medicinal chemistry. Potent ligands are currently in different stages of clinical trials for the antiangiogenic therapy of cancer and agerelated macula degeneration (AMD). The subtype a5b1has recently been drawn into the focus of research because of its genuine role in angiogenesis. In our previous work we could demonstrate that the lack of structural information about the receptor could be overcome by a homology model based on the X-ray structure of the avb3 integrin subtype and the sequence similarities between both receptors. In this work, we describe the rational design and synthesis of high affinity alpha-5-beta-1binders, and the optimisation of selectivity against avb3 by means of extensive SAR studies and docking experiments. A first series of compounds based on the tyrosine scaffold resulted in affinities in the low and even subnanomolar range and selectivities of 400-fold against avb3. The insights about the structure–activity relationship gained from tyrosine-based ligands could be successfully transferred to ligands that bear an aza-glycine scaffold to yield alpha-5-beta-1 ligands with affinities of ~1nm and selectivities that exceed 104- fold. The ligands have already been successfully employed as selective alpha-5-beta-1ligand s in biological research and might serve as lead structures for antiangiogenic cancer therapy.