Pharmacophoric Modifications Lead to Superpotent αvβ3 Integrin Ligands with Suppressed α5β1 Activity
23-Mar-2014
J. Med. Chem., 2014, DOI: 10.1021/jm500092w, 57 (8), pp 3410–3417 published on 23.03.2014
J. Med. Chem., online article
J. Med. Chem., online article
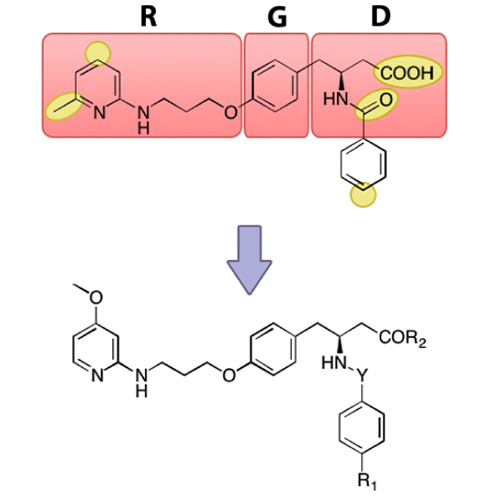
The selective targeting of the αvβ3 integrin subtype without affecting the structurally closely related receptor α5β1 is crucial for understanding the details of their biological and pathological functions and thus of great relevance for diagnostic and therapeutic approaches in cancer treatment. Here, we present the synthesis of highly active RGD peptidomimetics for the αvβ3 integrin with remarkable selectivity against α5β1. Incorporation of a methoxypyridine building block into a ligand scaffold and variation of different functional moieties led to αvβ3-antagonistic activities in the low nanomolar or even subnanomolar range. Furthermore, docking studies were performed to give insights into the binding modes of the novel compounds. The presented library comprises powerful ligands for specific addressing and blocking of the αvβ3 integrin subtype, thereby representing privileged tools for integrin-based personalized medicine.