Non-equilibrium hydrogen exchange for determination of H-bond strength and water accessibility in solid proteins
09-Apr-2017
Journal of Biomolecular NMR, Volume 68, Issue 1, pp 7–17, DOI 10.1007/s10858-017-0110-0
Journal of Biomolecular NMR, online article
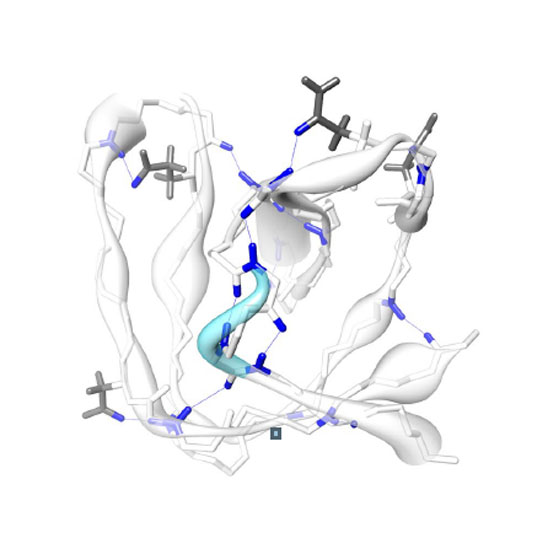
We demonstrate measurement of non-equilibrium backbone amide hydrogen–deuterium exchange rates (HDX) for solid proteins. The target of this study are the slowly exchanging residues in solid samples, which are associated with stable secondary-structural elements of proteins. These hydrogen exchange processes escape methods measuring equilibrium exchange rates of faster processes. The method was applied to a micro-crystalline preparation of the SH3 domain of chicken α-spectrin. Therefore, from a 100% back-exchanged micro-crystalline protein preparation, the supernatant buffer was exchanged by a partially deuterated buffer to reach a final protonation level of approximately 20% before packing the sample in a 1.3 mm rotor. Tracking of the HN peak intensities for 2 weeks reports on site-specific hydrogen bond strength and also likely reflects water accessibility in a qualitative manner. H/D exchange can be directly determined for hydrogen-bonded amides using 1H detection under fast magic angle spinning. This approach complements existing methods and provides the means to elucidate interesting site-specific characteristics for protein functionality in the solid state.