Multiple N-Methylation by a Designed Approach Enhances Receptor Selectivity
01-Nov-2007
Journal of Medicinal Chemistry, 2007, 50 (24), 5878-81 published on 01.11.2007
Journal of Medicinal Chemistry, online article
Journal of Medicinal Chemistry, online article
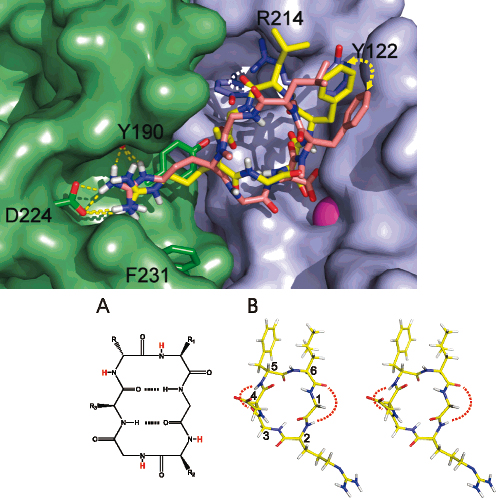
An unselective cyclic peptide integrin ligand was sequentially N-methylated by a designed approach, where only the externally oriented (solvent exposed) amide bonds were N-methylated. The N-methylation resulted in tremendous enhancement in selectivity among the different integrin receptor subtypes (alpha5beta1, alphavbeta3, and alphaIIbbeta3). Conformational and docking studies were performed, which suggested that the receptor selectivity is principally caused by reduced backbone flexibility due to N-methylation.