Immobilization of soluble protein complexes in MAS solid-state NMR: Sedimentation versus viscosity
15-Mar-2016
Solid State Nuclear Magnetic Resonance, Volumes 76–77, Pages 7–14, http://dx.doi.org/10.1016/j.ssnmr.2016.03.005
Solid State Nuclear Magnetic Resonance, online article
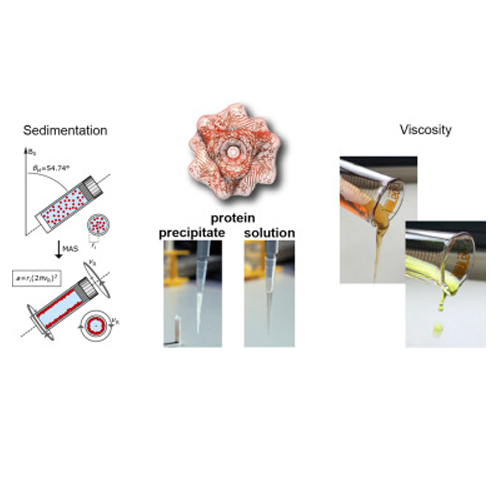
In recent years, MAS solid-state NMR has emerged as a technique for the investigation of soluble protein complexes. It was found that high molecular weight complexes do not need to be crystallized in order to obtain an immobilized sample for solid-state NMR investigations. Sedimentation induced by sample rotation impairs rotational diffusion of proteins and enables efficient dipolar coupling based cross polarization transfers. In addition, viscosity contributes to the immobilization of the molecules in the sample. Natural Deep Eutectic Solvents (NADES) have very high viscosities, and can replace water in living organisms. We observe a considerable amount of cross polarization transfers for NADES solvents, even though their molecular weight is too low to yield significant sedimentation. We discuss how viscosity and sedimentation both affect the quality of the obtained experimental spectra. The FROSTY/sedNMR approach holds the potential to study large protein complexes, which are otherwise not amenable for a structural characterization using NMR. We show that using this method, backbone assignments of the symmetric proteasome activator complex (1.1 MDa), and high quality correlation spectra of non-symmetric protein complexes such as the prokaryotic ribosome 50S large subunit binding to trigger factor (1.4 MDa) are obtained.