FOSL1 controls the assembly of endothelial cells into capillary tubes by direct repression of αv and β3 integrin transcription.
14-Jan-2013
Molecular and Cellular Biology, 2013, doi: 10.1128/MCB.01054-12, published on 14.01.2013
Molecular and Cellular Biology, online article
Molecular and Cellular Biology, online article
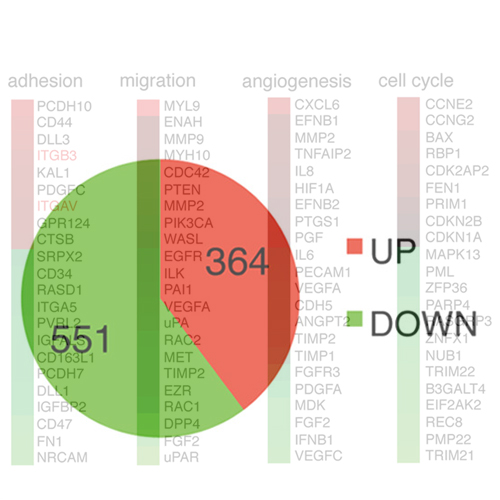
To form three-dimensional capillary tubes endothelial cells must establish contacts with the extracellular matrix that provides signals for their proliferation, migration, and differentiation. The transcription factor Fosl1 plays a key role in the vasculogenic and angiogenic processes, as Fosl1 knockout embryos die with vascular defects in extra-embryonic tissues. Here we show that Fosl1-/- embryonic stem cells differentiate to endothelial cells but fail to correctly assemble into primitive capillaries and to form tube-like structures. FOSL1 silencing affects in vitro angiogenesis, increases cell adhesion, and decrease cell mobility of primary human endothelial cells (HUVEC). We further show that FOSL1 is a repressor of αv and β3 integrins expression, and the down modulation of αvβ3 rescues the angiogenic phenotype in FOSL1-silenced HUVEC, while the ectopic expression of αvβ3 alone reproduces the phenotypic alterations induced by FOSL1 knockdown. FOSL1 represses the transcription of both αv and β3 integrin genes by binding together with JUND to their proximal promoter via the transcription factor SP1. These data suggest that FOSL1-dependent negative regulation of αvβ3 expression on endothelial cells is required for endothelial assembly into vessel structures.