Epigallocatechin-3-gallate preferentially induces aggregation of amyloidogenic immunoglobulin light chains
27-Jan-2017
Scientific Reports, volume 7, Article number: 41515, doi:10.1038/srep41515
Scientific Reports, online article
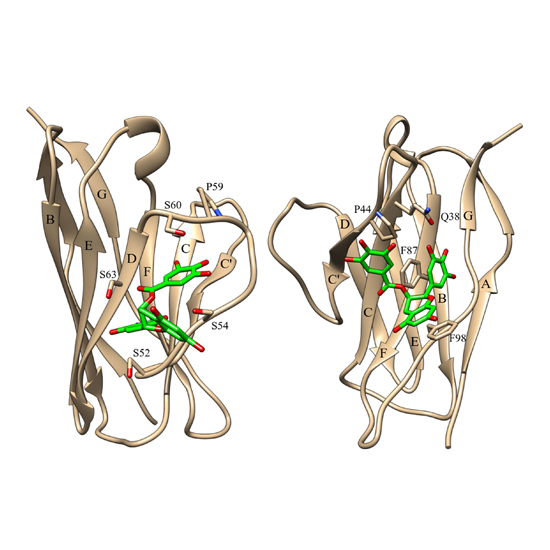
Antibody light chain amyloidosis is a rare disease caused by fibril formation of secreted immunoglobulin light chains (LCs). The huge variety of antibody sequences puts a serious challenge to drug discovery. The green tea polyphenol epigallocatechin-3-gallate (EGCG) is known to interfere with fibril formation in general. Here we present solution- and solid-state NMR studies as well as MD simulations to characterise the interaction of EGCG with LC variable domains. We identified two distinct EGCG binding sites, both of which include a proline as an important recognition element. The binding sites were confirmed by site-directed mutagenesis and solid-state NMR analysis. The EGCG-induced protein complexes are unstructured. We propose a general mechanistic model for EGCG binding to a conserved site in LCs. We find that EGCG reacts selectively with amyloidogenic mutants. This makes this compound a promising lead structure, that can handle the immense sequence variability of antibody LCs.