Enantiomeric Cyclic Peptides with Different Caco-2 Permeability Suggest Carrier-Mediated Transport
27-Apr-2015
Chemistry - A European Journal, 2015, DOI: 10.1002/chem.201501270, Volume 21, Issue 22, pages 8023–8027, published on 27.04.2015
Chemistry - A European Journal, online article
Chemistry - A European Journal, online article
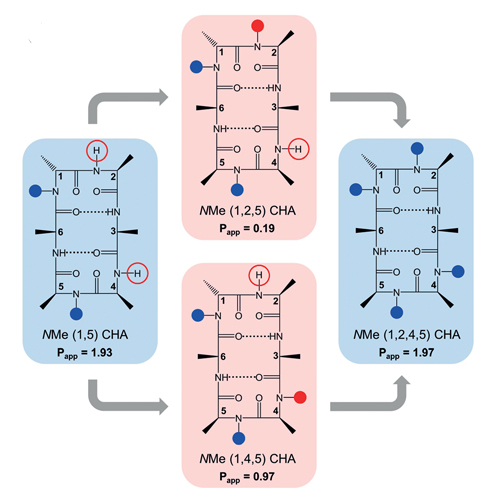
Recently, oral absorption of cyclic hexapeptides was improved by N-methylation of their backbone amides. However, the number and position of N-methylations or of solvent exposed NHs did not correlate to intestinal permeability, measured in a Caco-2 model. In this study, we investigate enantiomeric pairs of three polar and two lipophilic peptides to demonstrate the participation of carrier-mediated transporters. As expected, all the enantiomeric peptides exhibited identical lipophilicity (logD7.4) and passive transcellular permeability determined by the parallel artificial membrane permeability assay (PAMPA). However, the enantiomeric polar peptides exhibited different Caco-2 permeability (Papp) in both directions a–b and b–a. The same trend was observed for one of the lipophilic peptide, whereas the second lipophilic enantiomer pair showed identical Caco-2 permeability (within the errors). These findings provide the first evidence for the involvement of carrier-mediated transport for peptides, especially for those of polar nature.