Cytosolic Viral Sensor RIG-I Is a 5′-Triphosphate–Dependent Translocase on Double-Stranded RNA
20-Feb-2009
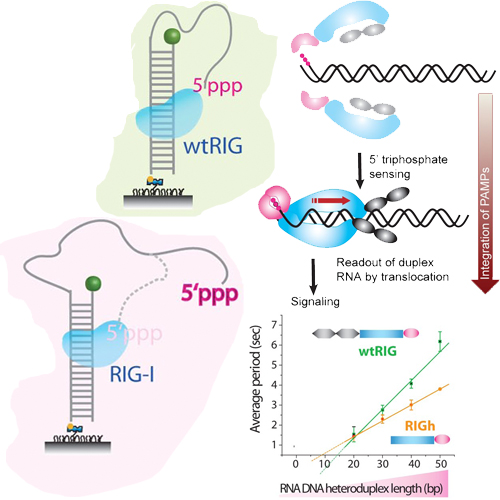
RIG-I is a cytosolic multi-domain protein that detects viral RNA and elicits an antiviral immune response. Two N-terminal caspase activation and recruitment domains (CARDs) transmit the signal and the regulatory domain prevents signaling in the absence of viral RNA. 5’-triphosphate and double stranded (ds) RNA are two molecular patterns that enable RIG-I to discriminate pathogenic from self-RNA. However, the function of the ATPase domain that is also required for activity is less clear. Using single-molecule fluorescence assays we discovered a robust, ATP-powered dsRNA translocation activity of RIG-I. The CARDs dramatically suppress translocation in the absence of 5’-triphosphate and the activation by 5’-triphosphate triggers RIG-I to translocate preferentially on dsRNA in cis. This functional integration of two RNA molecular patterns may provide a means to specifically sense and counteract replicating viruses.