Assignment strategies for aliphatic protons in the solid-state in randomly protonated proteins
04-Dec-2011
Journal of Biomolecular NMR, 2011, DOI: 10.1007/s10858-011-9591-4, Volume 52, Number 1, 31-39 published on 04.12.2011
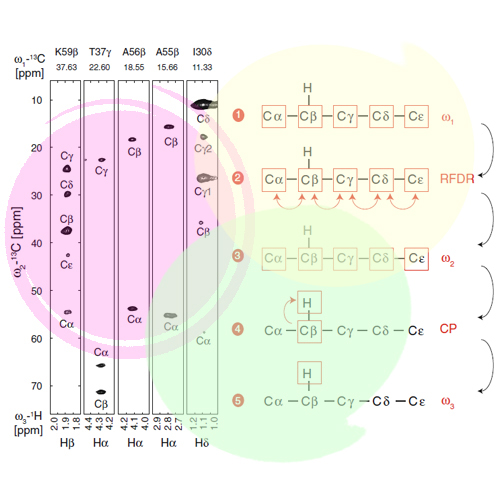
Biological solid-state nuclear magnetic resonance spectroscopy developed rapidly in the past two decades and emerged as an important tool for structural biology. Resonance assignment is an essential prerequisite for structure determination and the characterization of motional properties of a molecule. Experiments, which rely on carbon or nitrogen detection, suffer, however, from low sensitivity. Recently, we introduced the RAP (Reduced Adjoining Protonation) labeling scheme, which allows to detect backbone and sidechain protons with high sensitivity and resolution. We present here a 1H-detected 3D (H)CCH experiment for assignment of backbone and sidechain proton resonances. Resolution is significantly improved by employing simultaneous 13CO and 13Cβ J-decoupling during evolution of the 13Cα chemical shift. In total, ~90% of the 1Hα-13Cα backbone resonances of chicken α-spectrin SH3 could be assigned.