A Conformational Switch Underlies ClpP Protease Function
04-May-2011
Angewandte Chemie, 2011, DOI: 10.1002/anie.201100666, Volume 50, Issue 25, pages 5749–5752 published on 04.05.2011
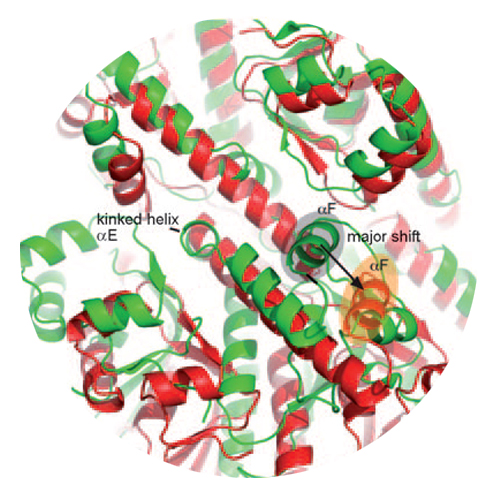
The barrel-shaped serine protease ClpP degrades misfolded, damaged, and regulatory proteins. Substrate proteins enter the ClpP barrel through the two axial pores, but it is unclear how the peptide products exit the barrel. Here we report the structure of ClpP from Staphylococcus aureus, which reveals a previously unobserved compressed state of the barrel. A conformational switch in the active center “handle region” results in closure of the active sites and opening of equatorial pores. Conserved residues in the handle region underlie the conformational switch and are functionally essential although they are not part of the active sites. These results are consistent with processive cycling of ClpP between an extended state with open active sites and closed equatorial pores, and a compressed state with closed active sites and open pores for product release.