Infrared Studies of Small Azobenzene Peptides: Unexpectedly Slow Reactions on the Time Range of Minutes
10-Aug-2007
J. Phys. Chem. B, 2007, 111, 10481-86 published on 10.08.2007
The Journal of Physical Chemistry B, online article
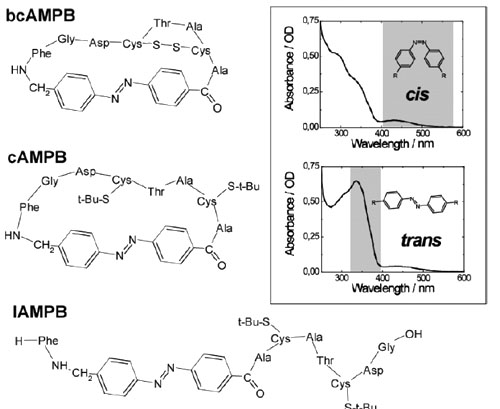
Infrared absorption experiments on light-triggered azobenzene peptides have been performed below and above the freezing point of the solvent dimethyl sulfoxide (DMSO). Even 20 K below the freezing point, illumination of the azobenzene chromophore resulted in IR absorption changes indicative of light-induced structural rearrangements of the peptide. In addition, new conformational states could be found at low temperature, which involve the formation of additional hydrogen bonds. In one sample the new low-temperature state survived melting and reheating and disappeared at 298 K only on the time scale of 10 min. The observations indicate that at low temperatures the peptide, together with traces of water present in the sample, forms a shell in the vicinity of the chromophore that facilitates internal motion even in the rigid cage of frozen DMSO.