Insights into the Binding of Pyridines to the Iron−Sulfur Enzyme IspH
09-May-2014
J. Am. Chem. Soc, 2014, DOI: 10.1021/ja501127j, 136 (22), pp 7926–7932 published on 09.05.2014
J. Am. Chem. Soc., online article
J. Am. Chem. Soc., online article
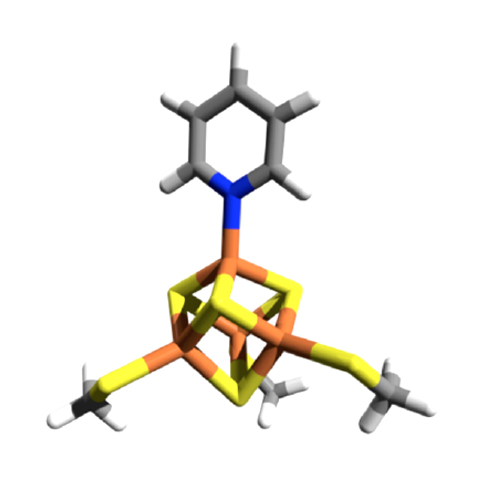
(E)-1-Hydroxy-2-methylbut-2-enyl 4-diphosphate reductase (IspH) is a [Fe4S4] cluster-containing enzyme involved in isoprenoid biosynthesis in many bacteria as well as in malaria parasites and is an important drug target. Several inhibitors including amino and thiol substrate analogues, as well as acetylene and pyridine diphosphates, have been reported. Here, we investigate the mode of binding of four pyridine diphosphates to Escherichia coli IspH by using X-ray crystallography. In three cases, one of the iron atoms in the cluster is absent, but in the structure with (pyridin-3-yl)methyl diphosphate, the most potent pyridine-analogue inhibitor reported previously, the fourth iron of the [Fe4S4] cluster is present and interacts with the pyridine ring of the ligand. Based on the results of quantum chemical calculations together with the crystallographic results we propose a side-on η2 coordination of the nitrogen and the carbon in the 2-position of the pyridine ring to the unique fourth iron in the cluster, which is in the reduced state. The X-ray structure enables excellent predictions using density functional theory of the 14N hyperfine coupling and quadrupole coupling constants reported previously using HYSCORE spectroscopy, as well as providing a further example of the ability of such [Fe4S4]-containing proteins to form organometallic complexes.