Assembly-induced folding regulates interleukin 12 biogenesis and secretion
21-Mar-2017
JBC, doi: 10.1074/jbc.M117.782284
JBC, online article
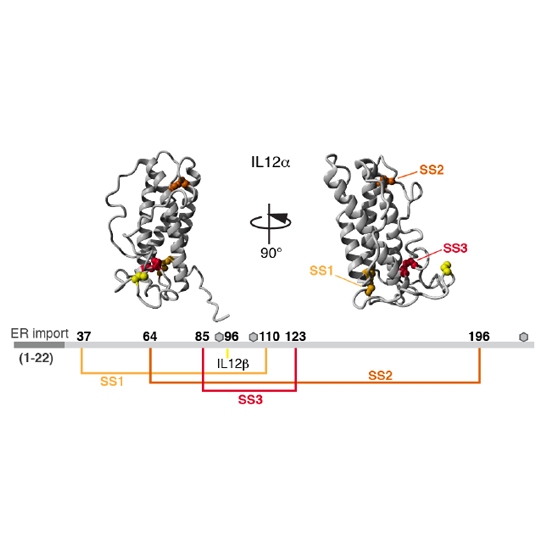
Members of the interleukin 12 (IL12) family perform essential functions in immunoregulation by connecting innate and adaptive immunity and are emerging therapeutic targets. They are unique among other interleukins in forming heterodimers that arise from extensive subunit sharing within the family, leading to the production of at least four functionally distinct heterodimers from only five subunits. This raises important questions about how the assembly of IL12 family members is regulated and controlled in the cell. Here, using cell biological approaches, we have dissected basic principles that underlie the biogenesis of the familys founding member, IL12. Within the native IL12 heterodimer, composed of IL12α and IL12β, IL12α possesses three intramolecular and one intermolecular disulfide bridges. We show that in isolation IL12α fails to form its native structure but instead misfolds, forming incorrect disulfide bonds. Co-expression of its β-subunit inhibits misfolding and thus allows secretion of biologically active heterodimeric IL12. On the basis of these findings, we identified the disulfide bonds in IL12α that are critical for assembly-induced secretion and biological activity of IL12 versus misfolding and degradation of IL12α. Surprisingly, two of the three disulfide bridges in IL12α are dispensable for IL12 secretion, stability and biological activity. Extending our findings, we show that misfolding also occurs for IL23α, another IL12 family protein. Our results indicate that assembly-induced folding is key in IL12 family biogenesis and secretion. The identification of essential disulfide bonds that underlie this process lays the basis for a simplified yet functional IL12 cytokine.