Heat shock protein 90’s mechanochemical cycle is dominated by thermal fluctuations
03-Jan-2012
PNAS, 2011, doi: 10.1073/pnas.1107930108, vol. 109, 161-166 published on 03.01.2012
PNAS, online article
PNAS, online article
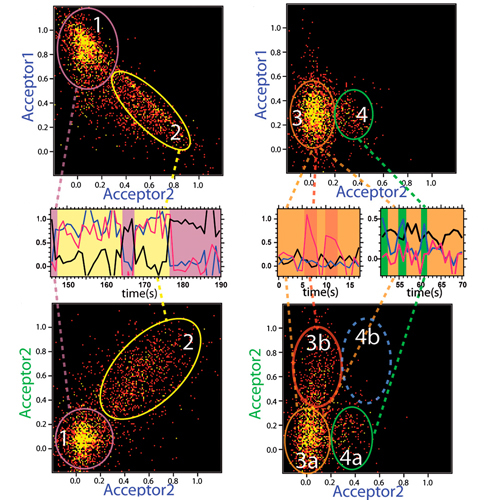
The molecular chaperone and heat shock protein 90 (Hsp90) exists mainly as a homodimer in the cytoplasm. Each monomer has an ATPase in its N-terminal domain and undergoes large conformational changes during Hsp90’s mechanochemical cycle. The threecolor single-molecule assay and data analysis presented in the following allows one to observe at the same time nucleotide binding and the conformational changes in Hsp90. Surprisingly, and completely unlike the prior investigated systems, nucleotides can bind to the N-terminally open and closed state without strictly forcing the protein into a specific conformation. Both the transitions between the conformational states and the nucleotide binding/ unbinding are mainly thermally driven. Furthermore, the two ATP binding sites show negative cooperativity; i.e., nucleotides do not bind independently to the two monomers.We thus reveal a picture of how nucleotide binding and conformational changes are connectedin the molecular chaperone Hsp90, which has far-ranging conseque nces for its function and is distinct from previously investigated motor proteins.