Evidence for a Broad Transition-State Ensemble in Calmodulin Folding from Single-Molecule Force Spectroscopy
29-Mar-2010
Angewandte Chemie International Edition, 2010, DOI: 10.1002/anie.200905747, Volume 49, Issue 19, pages 3306–3309, published on 29.03.2010
Angewandte Chemie International Edition, online article
Angewandte Chemie International Edition, online article
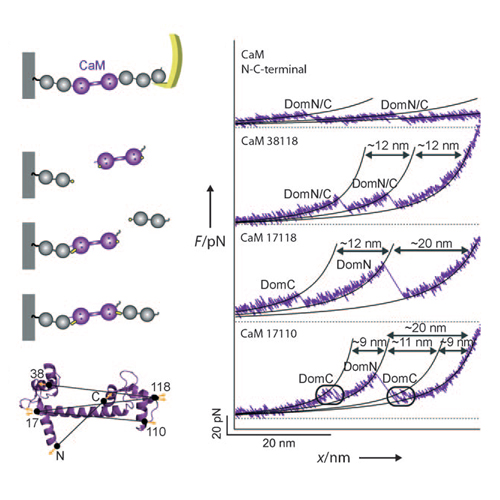
In recent years, single-molecule force spectroscopy has provided a wealth of insights into protein folding and unfolding.1–7 Using mechanical force as a denaturant offers distinct advantages over thermal or chemical denaturation, such as the possibility to locally probe the folding free-energy landscape8 or to actively steer the unfolding pathway of proteins.9 However, it has been argued that the unfolding pathways that are probed in single-molecule force spectroscopy are likely to be very different from the pathways studied by chemical denaturation,10 which would limit or exclude any comparison between single-molecule force spectroscopy experiments and conventional folding techniques.