Expression of the anti-amyloidogenic secretase ADAM10 is suppressed by its 5′-untranslated region
26-Mar-2010
J. Biol. Chem., 2010, doi: 10.1074/jbc.M110.110742 published on 26.03.2010
The Journal of Biological Chemistry, online article
The Journal of Biological Chemistry, online article
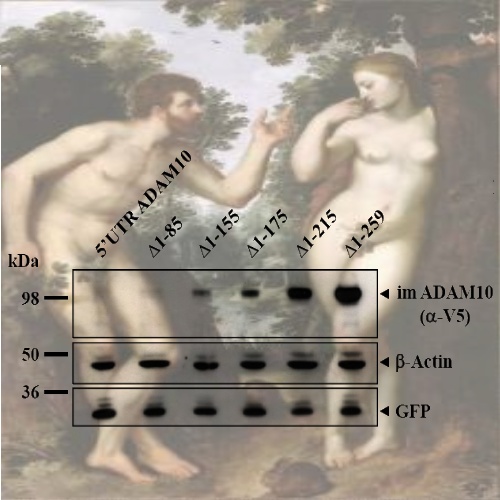
Proteolytic processing of the amyloid precursor protein (APP) by α-secretase prevents formation of the amyloid β-peptide (Aβ), which is the main constituent of amyloid plaques in brains of Alzheimer′s disease (AD) patients. α-Secretase activity is decreased in AD and overexpression of the α-secretase ADAM10 (a disintegrin and metalloprotease) in an AD animal model prevents amyloid pathology. ADAM10 has a 444 nucleotides long,very GC-rich 5′ untranslated region (5′UTR) with two upstream open reading frames. Since similar properties of 5′UTRs are found in transcripts of many genes, which are regulated by translational control mechanisms, we asked whether ADAM10 expression is translationally controlled by its 5′UTR. We demonstrate that the 5′UTR of ADAM10 represses the rate of ADAM10 translation. In the absence of the 5′UTR we observed a significant increase of ADAM10 protein levels in HEK293 cells while mRNA levels were not changed. Moreover, the 5′UTR of ADAM10 inhibits translation of a luciferase reporter in an in vitro transcription / translation assay. Successive deletion of the first half of the ADAM10 5′UTR revealed a striking increase in ADAM10 protein expression in HEK293 cells, suggesting that this part of the 5′UTR contains inhibitory elements for translation. Moreover, we detect an enhanced α-secretase activity and consequently reduced Aβ levels in conditioned media of HEK293-APP cells expressing a 5′UTR-ADAM10 deletion construct lacking the first half of the 5′UTR. Thus, we provide evidence that the 5′UTR of ADAM10 may have an important role for posttranscriptional regulation of ADAM10 expression and consequently Aβ production.